Nearly 2,000 HeartWare Ventricular Assist Systems (VAS) are being recalled because of potentially faulty power supply connectors. The defect could lead to serious injury or death in the event that 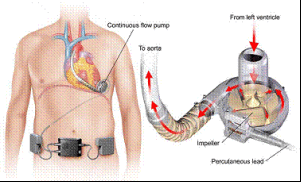 power failure causes the entire system to stop working.
power failure causes the entire system to stop working.
The Recall
The FDA issued a Class I recall for certain VAS devices after receiving numerous reports of malfunctions. Luckily, most of the failures did not result in any damage, but there was at least one serious injury reported due to a power failure. A “Class I” recall means that the FDA thinks that there is a “reasonable probability” that the continued use of the product will lead to serious health consequences or even death. Obviously, this is a recall to take very seriously should you use VAS. Mechanically, the main issue is that power connector ports tend to wear down over time. Should they become worn down enough, the power supply would lose its connection to the system, effectively rendering the VAS useless. Patients have reported that the power supply connectors have become twisted or bent, which prevents them from connecting the device control unit to the VAS itself. You know how when you’re vacuuming and you round a corner too quickly, ripping the cord out of the wall and bending up the power cord prongs? That’s what is going on here. The stakes are just a little higher.
This is such a big deal because VAS devices are used in patients who are awaiting heart transplants due to end-stage left ventricular heart failure. The device is designed to pump blood to the rest of the body by using an artificial pump placed around the heart. The system is then customized to accommodate the speed and function required for each individual. Think of a VAS as a pacemaker that not only kick starts heart function, but then does some of the heart’s job as well.
HeartWare “Urgent Device Correction”
The specific devices being recalled include all VAS devices with the product codes 1101 and 1103, which were manufactured by HeartWare of Miami Lakes. They were sold all across the USA from January 2008 to March of this year. HeartWare picked up on the issue too, issuing an “Urgent Medical Device Correction” to users. They even said that they will replace the defective controllers by July 2016. However, their procedure has a few more moving parts than the FDA recall: healthcare providers have to identify all patients affected by the recall and warn them via traceable mail. The patients have to make an appointment with their health care professional, who will inspect the power supply and advise the patient accordingly. HeartWare has set up a 24-hour clinical support line for any inquiries related to their products. You can call them at 1-888-494-6365 or email them at FSCA@heartware.com with concerns. Plus, the FDA wants your help with reporting any adverse reactions or quality concerns related to HeartWare’s products.
Previous HeartWare Recalls
Last July, HeartWare recalled a similar device after two people died and four more were seriously injured from power connection issues. This didn’t sit well with the feds, who decided to take a look at their Miami Lakes Facility, noting some serious deficiencies involving their process for making sure that their products, you know, work. They were issued a warning following that inspection, which appraised the company of the fact that their practices were sub-par compared to other medical device manufacturers. This whole thing is just a mess.
 Lawsuit Information Center
Lawsuit Information Center

