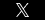A man from Tennessee has filed a lawsuit against Boehringer Ingelheim Pharmaceuticals, Inc., the maker of Pradaxa. The drug company is being sued by the man who claims his father died as a result of side effects he experienced while taking the blood-thinning drug. Melvin Giles, Jr. sued Boehringer Ingelheim in the U.S. District Court for the Middle District of Tennessee.
According to the suit, Giles’ father was prescribed the drug as a blood thinner. The medication is usually used to reduce the chance of blood clots developing and leading to a stroke in patients who present special risks for such problems. Giles’ father was given the medication in February of 2011 and in June of that same year suffered severe internal bleeding and died as a result. His son claims that the bleeding he suffered was the result of Pradaxa.
Giles claims that the drug maker either knew or should have known about the potential for the dug to cause such serious complications. The suit cites as proof the 932 incidents reported to the FDA between October 2010 and March 2011 related to side effects of the medication. Some 120 of these reports involved death from Pradaxa bleeding, and 500 were severe and life-threatening bleeding events related to the medication. These numbers have gone on to spike and according to a new report from the Institute for Safe Medicine Practices (ISMP), the U.S. Food & Drug Administration received 3,781 adverse event reports associated with Pradaxa in 2011, more than were associated with any other drug the agency monitors.
Pradaxa also led in reports of deaths (541), hemorrhage (2,367), kidney failure (291) and stroke (644), according to ISMP’s most recent report. Pradaxa was also a suspected cause of 15 reports of liver injury made to the FDA.
The problems with Pradaxa began in 2011 when the drug became the subject of an investigation in New Zealand after as many as five elderly patients died as a result of internal bleeding. Another 36 patients reportedly suffered similar bouts of serious internal bleeding but managed to survive. These reports came on the heels of similar news in Japan that regulators asked Boehringer Ingelheim to notify doctors about potentially deadly bleeding associated with the drug.
Pradaxa was heavily marketed when it was first introduced as a safer alternative to traditional blood thinners such as warfarin. The benefit was that Pradaxa does not react with certain foods in the same way that warfarin does which can increase the likelihood of bleeding. The problem is that warfarin bleeding can be controlled and even stopped by administering vitamin K. The danger of Pradaxa is that there is currently no known antidote for Pradaxa-caused bleeding, making injuries associated with the product far more deadly.
If you or someone you know have been injured and you would like to discuss your case with an attorney, please contact one of our experienced medical malpractice attorneys today for a free consultation at (267) 809-8250.
Source: “Wrongful Death Suit Filed After Pradaxa Bleeding Death,” by Elise Kramer, published at InjuryLawyer-News.com.
See Our Related Blog Posts:
$1.6M Delaware Jury Verdict Following Wrongful Prescription of Sotalol
Stevens Johnson Syndrome
 Philadelphia Medical Malpractice Lawyer Blog
Philadelphia Medical Malpractice Lawyer Blog